Reaction Conditions for Chemical coupling (S9150)
We recommend that coupling reactions between BG-PEG-NH2 and NHS esters or activated carboxylic acids take place in anhydrous N,N-dimethyl formamide (DMF) using triethylamine as a base.
Typical reactions use approximately equimolar quantities of the BG-PEG-NH2 and the desired NHS-label at 5-20 mM final concentration in the presence of a 1.5 fold molar excess of triethylamine in DMF. The reaction is generally performed at 30°C overnight.
Example Reaction: Coupling of BG-PEG-NH2 to (+)-biotin-N-hydroxysuccinimide ester:
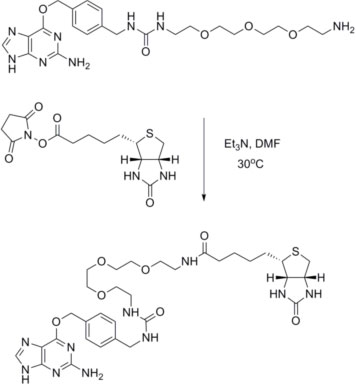
The purification strategy will depend on the label used and the reaction scale. Good results have been obtained with both HPLC and silica get chromatography.

